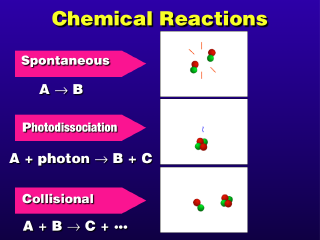
The energy of a photon is used to activate the photodissociation reaction (also known as photolysis). The increased energy content of the molecule that absorbed the photon leads to its breakup. There are other photochemical reactions that are like this but may not result in a molecular breakup into new compounds--in photoionization, an atom or molecule absorbs a photon of light and an electron is thrown off, leaving the substance unchanged except that it has a net positive electronic charge from having lost the electron(s). The resulting substance is an ion of the original substance.
Collisional reactions require the reactants to touch each other (collide). If more than two reactants are required, all of the reacting bodies must collide simultaneously at the same point in space. In gases, the likelihood of this happening for three or more reactant bodies is pretty small, given the random motions of molecules in a gas. Not that it rarely happens; in the lower atmosphere, the overall concentration of gases is much higher than aloft, so the occurrence of three-body collisions is much more frequent near the ground than up high in the atmosphere.