 Minor
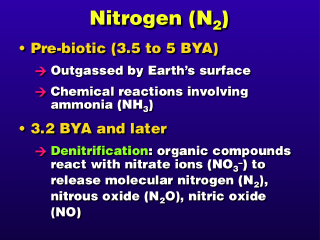
outgas products from the Earth included molecular nitrogen (N2) and
ammonia (NH3). Before oxygen became a major constituent of the Earth's atmosphere,
ammonia absorbed some of the solar ultraviolet radiation, broke up into component
atoms, and recombined into other compounds, including molecular nitrogen.
Minor
outgas products from the Earth included molecular nitrogen (N2) and
ammonia (NH3). Before oxygen became a major constituent of the Earth's atmosphere,
ammonia absorbed some of the solar ultraviolet radiation, broke up into component
atoms, and recombined into other compounds, including molecular nitrogen.
After lifeforms evolved, anaerobic
bacteria reacted organic compounds with nitrates occurring in the environment
and within their bodies. This chemical process is called denitrification and was the source of most of the atmosphere's molecular nitrogen.
Because molecular nitrogen is fairly
unreactive in the Earth's atmosphere, so it has a very long lifetime, and the
source rate of nitrogen from denitrification was greater than the removal rate
of nitrogen in the early days, the nitrogen content of the atmosphere built
up to a very high mixing ratio over the next few billion years.
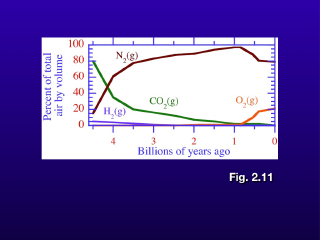 This plot shows the overall evolution of the major gas concentrations over the time of the Earth's existence.
This plot shows the overall evolution of the major gas concentrations over the time of the Earth's existence.



 Minor
outgas products from the Earth included molecular nitrogen (N2) and
ammonia (NH3). Before oxygen became a major constituent of the Earth's atmosphere,
ammonia absorbed some of the solar ultraviolet radiation, broke up into component
atoms, and recombined into other compounds, including molecular nitrogen.
Minor
outgas products from the Earth included molecular nitrogen (N2) and
ammonia (NH3). Before oxygen became a major constituent of the Earth's atmosphere,
ammonia absorbed some of the solar ultraviolet radiation, broke up into component
atoms, and recombined into other compounds, including molecular nitrogen. This plot shows the overall evolution of the major gas concentrations over the time of the Earth's existence.
This plot shows the overall evolution of the major gas concentrations over the time of the Earth's existence.