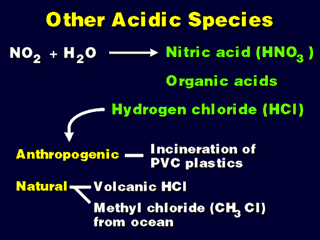
 In Los Angeles, there is much more NOx than SOx in the air, so acid deposition
here is likely to be nitric acid-based. Note that NO2 can be converted
to HNO3 in similar ways as SOx becomes H2SO4; namely, oxidation while dissolved
in water, or through reaction with water vapor in the atmosphere.
In Los Angeles, there is much more NOx than SOx in the air, so acid deposition
here is likely to be nitric acid-based. Note that NO2 can be converted
to HNO3 in similar ways as SOx becomes H2SO4; namely, oxidation while dissolved
in water, or through reaction with water vapor in the atmosphere.
HCl (hydrogen chloride) is also known as hydrochloric acid when dissolved
in water. The natural source is actually quite large compared to the anthropogenic
source, but the lifetime in the troposphere is quite short for both (both HCl
and methyl chloride are present over the ocean, and quickly disappear back into
the ocean water) so the risk of acid deposition damage from HCl is small. HCl
has a significant lifetime in the stratosphere, and can potentially reduce the
ozone concentration up there through the release of the chlorine atom from the
HCl, but the extent of this ozone depletion has been greatly overestimated in
the past (HCl at these altitudes were probably directly injected by large volcanic
eruptions).
Organic acids, such as formic acid (excreted by ants so they can mark a trail)
form such a small contribution to the overall acid deposition problem that their
effect on the environment is practically negligible.



 In Los Angeles, there is much more NOx than SOx in the air, so acid deposition
here is likely to be nitric acid-based. Note that NO2 can be converted
to HNO3 in similar ways as SOx becomes H2SO4; namely, oxidation while dissolved
in water, or through reaction with water vapor in the atmosphere.
In Los Angeles, there is much more NOx than SOx in the air, so acid deposition
here is likely to be nitric acid-based. Note that NO2 can be converted
to HNO3 in similar ways as SOx becomes H2SO4; namely, oxidation while dissolved
in water, or through reaction with water vapor in the atmosphere.