
 |
 |
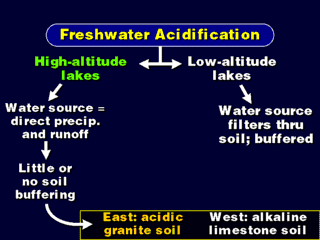
| High-altitude lakes do not usually have the benefit of melting snow to flush out the polluted water in the springtime. All of their water comes from nearby runoff and precipitation. | This is one of the Adirondack lakes. |
 |
 |
| Western soil contains a lot of limestone, a natural alkaline substance which neutralizes acids. So, not only is the West in a spot that is free from having acid sources upwind, they have a natural protection system. | Eastern soils are more granite-like, and do not have the acid-buffering qualities of limestone. In this scene, the roadway is barren because the soil is acidic. |
 |
The photo at the lower left shows the very clear water in an Adirondack lake. The acidity of the water has killed off most of the aquatic life, including algae and microbes. Normally, the water would ber very murky from whatever lives in the water. The photo at the lower right shows a collection of fallen leaves in an Adirondack lake. Normally, these leaves would decompose quickly and add to the murkiness of the water and to the sediment at the bottom. But because there are very few bacteria and fungi in the acidified water, the leaves remain fairly well-preserved in the water. |
 |
 |
 These fish gills are clogged with an aluminum oxide deposit. The high levels
of aluminum ions in the water is a result of the low pH.
These fish gills are clogged with an aluminum oxide deposit. The high levels
of aluminum ions in the water is a result of the low pH.