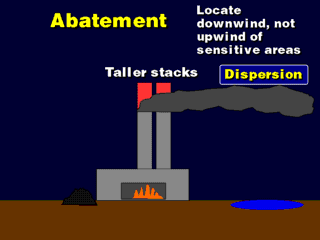
 This does not change the amount of SOx coming out of the stacks, but helps
spread it around to a larger region so the concentration of the acids are too
low to detect easily. In the United States, it is best to put coal-fired power
plants right along the Eastern Coast, where the smoke plumes will disperse over
the Atlantic Ocean, which can easily take the SOx.
This does not change the amount of SOx coming out of the stacks, but helps
spread it around to a larger region so the concentration of the acids are too
low to detect easily. In the United States, it is best to put coal-fired power
plants right along the Eastern Coast, where the smoke plumes will disperse over
the Atlantic Ocean, which can easily take the SOx.
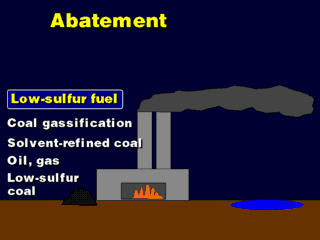 Since "dirty" coal, found in the U.S., is the main source of SOx,
we could find alternative fuels that are low in sulfur, or remove the sulfur
from existing, high-sulfur coal.
Since "dirty" coal, found in the U.S., is the main source of SOx,
we could find alternative fuels that are low in sulfur, or remove the sulfur
from existing, high-sulfur coal.
Oil and gas are naturally low in sulfur compared to coal, so a lot of
power plants have been converted to these fuels. A side benefit is that emissions
of particulates (soot) and other toxic, organic compounds has been reduced.
Anthracite is a coal with 1% or less sulfur content. It is rather expensive,
so it is not used a lot.
Solvent-refined coal (SRC) is coal which has been dumped into a carbon
solvent (anthracene), which dissolves the carbon element (thte part that we
want to burn) and leaves the sulfur and other minerals behind (which is the
waste). The solid materials are filtered out and either put in a toxic waste
dump or recycled; the carbon-laced solvent is heated to evaporate the solvent
(the solvent fumes are recovered and condensed back into liquid, to be used
on another batch of coal) and the carbon dust is used in the furnace. This process
is pretty expensive to implement, so it is not used much unless heavily subsidized
by the government.
Coal gassification (CG) is a process where coal is heated in an oxygen-rich
environment. The carbon in the coal oxidizes into carbon monoxide (CO), which
is collected and burned just like natural gas. Meanwhile, the sulfur does not
oxidize during the gassification process, so it is left behind to be recycled
or dumped. This process is extremely expensive, and the energy value of the
CO gas is much lower than real natural gas (methane, CH4), so we don't get much
heat out of this. This process is pretty much dead in the United States, except
for a couple of demonstration projects.
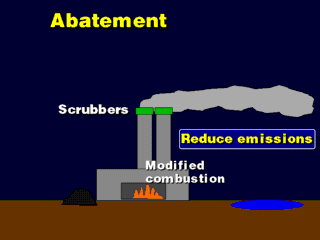 Scrubbers are devices mounted in the smokestack to clean the SOx emissions
from the smoke. This is accomplished by spraying water into the smoke (simulating
a washout process) or spraying a limestone-water slurry,
which washes out the SOx and chemically neutralizes it. The wastewater is collected
and shipped off to a chemicals plant to recycle the sulfuric acid; if a limestone
slurry is used, the resulting calcium sulfate is recycled into plaster and drywall
board. A disadvantage of the technique is that the water cools the smoke, so
we usually need to heat it up again to make it buoyant enough to flow out of
the smokestack. The main advantage of this technique is that it is very easy
to retrofit old smokestacks with scrubbers, so practically all stacks in the
U.S. have scrubbers.
Scrubbers are devices mounted in the smokestack to clean the SOx emissions
from the smoke. This is accomplished by spraying water into the smoke (simulating
a washout process) or spraying a limestone-water slurry,
which washes out the SOx and chemically neutralizes it. The wastewater is collected
and shipped off to a chemicals plant to recycle the sulfuric acid; if a limestone
slurry is used, the resulting calcium sulfate is recycled into plaster and drywall
board. A disadvantage of the technique is that the water cools the smoke, so
we usually need to heat it up again to make it buoyant enough to flow out of
the smokestack. The main advantage of this technique is that it is very easy
to retrofit old smokestacks with scrubbers, so practically all stacks in the
U.S. have scrubbers.
By modifying the combustion process, we can prevent the formation of SOx during
combustion. The usual technique is call Fluidized Bed Combustion (FBC),
where the coal is ground up, mixed with limestone dust, and blown into the furnace
over jets of air. This different from the usual combustion technique where chunks
of coal roll through the furnace on a conveyer belt, and the heat from the burning
coal rises up and heats water in pipes running through the furnace. The air
jets "fluidize" the coal-limestone mixture, forming a cloud in the
furnace. When the coal particles burn, the SOx is immediated reacted with the
particles of limestone floating around in the cloud, accomplishing the same
thing as a limestone scrubber but without the cooling effect of the water. In
fact, because the coal is burning in a cloud surrounding the water pipes, the
heat transfer is more efficient, so we get more energy value out of the coal
than with conventional burners. This allows us to lower the combustion temperature,
which results in less NOx being produced during the combustion process!
 If the environment is already acidified by smokestack emissions, clean-up
measures must be taken. This usually involves spreading limestone or some other
alkaline buffer around to neutralize the acids. Unfortunately, this requires
a lot of buffering material (tons), transported to areas difficult to access
with large trucks. And, if the smokestack emissions upwind are not corrected
for acidic emissions, this has to be repeated every year.
If the environment is already acidified by smokestack emissions, clean-up
measures must be taken. This usually involves spreading limestone or some other
alkaline buffer around to neutralize the acids. Unfortunately, this requires
a lot of buffering material (tons), transported to areas difficult to access
with large trucks. And, if the smokestack emissions upwind are not corrected
for acidic emissions, this has to be repeated every year.


 This does not change the amount of SOx coming out of the stacks, but helps
spread it around to a larger region so the concentration of the acids are too
low to detect easily. In the United States, it is best to put coal-fired power
plants right along the Eastern Coast, where the smoke plumes will disperse over
the Atlantic Ocean, which can easily take the SOx.
This does not change the amount of SOx coming out of the stacks, but helps
spread it around to a larger region so the concentration of the acids are too
low to detect easily. In the United States, it is best to put coal-fired power
plants right along the Eastern Coast, where the smoke plumes will disperse over
the Atlantic Ocean, which can easily take the SOx. Since "dirty" coal, found in the U.S., is the main source of SOx,
we could find alternative fuels that are low in sulfur, or remove the sulfur
from existing, high-sulfur coal.
Since "dirty" coal, found in the U.S., is the main source of SOx,
we could find alternative fuels that are low in sulfur, or remove the sulfur
from existing, high-sulfur coal. Scrubbers are devices mounted in the smokestack to clean the SOx emissions
from the smoke. This is accomplished by spraying water into the smoke (simulating
a
Scrubbers are devices mounted in the smokestack to clean the SOx emissions
from the smoke. This is accomplished by spraying water into the smoke (simulating
a  If the environment is already acidified by smokestack emissions, clean-up
measures must be taken. This usually involves spreading limestone or some other
alkaline buffer around to neutralize the acids. Unfortunately, this requires
a lot of buffering material (tons), transported to areas difficult to access
with large trucks. And, if the smokestack emissions upwind are not corrected
for acidic emissions, this has to be repeated every year.
If the environment is already acidified by smokestack emissions, clean-up
measures must be taken. This usually involves spreading limestone or some other
alkaline buffer around to neutralize the acids. Unfortunately, this requires
a lot of buffering material (tons), transported to areas difficult to access
with large trucks. And, if the smokestack emissions upwind are not corrected
for acidic emissions, this has to be repeated every year.