 The breakdown of cellulose occurs in the stomachs of cattle. This links the
production of methane gas (CH4) to food production (meat and milk). Decay
of dead organic material also produces methane gas. This occurs in bogs and
marshes, where murky water pools and harbors many microorganisms that break
down dead plant material in the marsh.
The breakdown of cellulose occurs in the stomachs of cattle. This links the
production of methane gas (CH4) to food production (meat and milk). Decay
of dead organic material also produces methane gas. This occurs in bogs and
marshes, where murky water pools and harbors many microorganisms that break
down dead plant material in the marsh.
Rice paddies are essentially
marshes, so anthropogenic CH4 is produced by this agricultural practice. For
other food crops, the decomposition of unharvested, waste plant material produces
methane. All this means is that as world population increases, the increased
food production results in increased CH4 emissions (and probably higher CO2
concentration as well, from the deforestation needed to support this food production).
This makes it difficult to regulate CH4 emissions, because there are few practical
substitutes to large-scale food production.
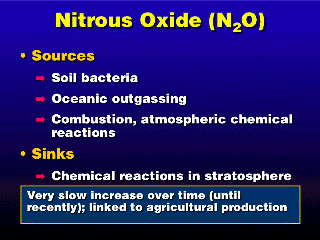 Nitrous oxide (N2O) emissions can be linked to agriculture just
like CH4, except now it's more widespread---it applies to just about anything
grown in soil. Soil bacteria, often living on the roots of plants (legumes,
such as beans, in particular) eat nitrate compounds in the soil and convert
them to N2O. In commercial agriculture, fertilizers (including that old standby,
steer manure) contain nitrates (helps the plant produce leafy green mass). Much
of the recent sudden increase in N2O emissions can be attributed to the use
of the nitrate fertilizers.
Nitrous oxide (N2O) emissions can be linked to agriculture just
like CH4, except now it's more widespread---it applies to just about anything
grown in soil. Soil bacteria, often living on the roots of plants (legumes,
such as beans, in particular) eat nitrate compounds in the soil and convert
them to N2O. In commercial agriculture, fertilizers (including that old standby,
steer manure) contain nitrates (helps the plant produce leafy green mass). Much
of the recent sudden increase in N2O emissions can be attributed to the use
of the nitrate fertilizers.
As with CH4, regulation of N2O emissions
will be difficult because without the nitrate fertilizers, the productivity
of agricultural lands may be too low to sustain the food production needed by
the large global population. Note that growing vegetables organically does not
significantly reduce the output of N2O--organic fertilizers are still supply
the same nitrate nutrients as chemical fertilizers, and vigorously growing plants
will result in the same N2O emissions no matter what the nitrogen source, since
it's all chemicals to plants anyway.
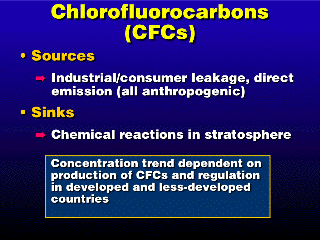 All chlorofluorocarbons (CFCs) are anthropogenic (except for a few
similar compounds that are not really true CFCs). Their long atmospheric lifetimes
(50-100 years) pose a potential problem, in that it allows the concentration
to build up before breakdown in the stratosphere can remove them (and then there
is a whole new set of problems from CFC breakdown in the stratosphere).
All chlorofluorocarbons (CFCs) are anthropogenic (except for a few
similar compounds that are not really true CFCs). Their long atmospheric lifetimes
(50-100 years) pose a potential problem, in that it allows the concentration
to build up before breakdown in the stratosphere can remove them (and then there
is a whole new set of problems from CFC breakdown in the stratosphere).
Modern countries like the U.S. have
agreed to limit and reduce the amount of CFCs manufactured and emitted to the
atmosphere, so the problem should take care of itself over time. Unfortunately,
developing countries are just now showing capacity to manufacture and/or use
these materials (for refrigeration, mostly), so in the near future, there may
still be a ramping-up of CFC concentration as these materials are used in these
regions. [One can suppose that in the absence of practical substitutes, CFCs
are linked to a necessity of modern life---refrigeration---and like the gases
that are linked to food production, it will be difficult to regulate something
that is essential to food, in this case preservation.]



 The breakdown of cellulose occurs in the stomachs of cattle. This links the
production of methane gas (CH4) to food production (meat and milk). Decay
of dead organic material also produces methane gas. This occurs in bogs and
marshes, where murky water pools and harbors many microorganisms that break
down dead plant material in the marsh.
The breakdown of cellulose occurs in the stomachs of cattle. This links the
production of methane gas (CH4) to food production (meat and milk). Decay
of dead organic material also produces methane gas. This occurs in bogs and
marshes, where murky water pools and harbors many microorganisms that break
down dead plant material in the marsh.  Nitrous oxide (N2O) emissions can be linked to agriculture just
like CH4, except now it's more widespread---it applies to just about anything
grown in soil. Soil bacteria, often living on the roots of plants (legumes,
such as beans, in particular) eat nitrate compounds in the soil and convert
them to N2O. In commercial agriculture, fertilizers (including that old standby,
steer manure) contain nitrates (helps the plant produce leafy green mass). Much
of the recent sudden increase in N2O emissions can be attributed to the use
of the nitrate fertilizers.
Nitrous oxide (N2O) emissions can be linked to agriculture just
like CH4, except now it's more widespread---it applies to just about anything
grown in soil. Soil bacteria, often living on the roots of plants (legumes,
such as beans, in particular) eat nitrate compounds in the soil and convert
them to N2O. In commercial agriculture, fertilizers (including that old standby,
steer manure) contain nitrates (helps the plant produce leafy green mass). Much
of the recent sudden increase in N2O emissions can be attributed to the use
of the nitrate fertilizers. All chlorofluorocarbons (CFCs) are anthropogenic (except for a few
similar compounds that are not really true CFCs). Their long atmospheric lifetimes
(50-100 years) pose a potential problem, in that it allows the concentration
to build up before breakdown in the stratosphere can remove them (and then there
is a whole new set of problems from CFC breakdown in the stratosphere).
All chlorofluorocarbons (CFCs) are anthropogenic (except for a few
similar compounds that are not really true CFCs). Their long atmospheric lifetimes
(50-100 years) pose a potential problem, in that it allows the concentration
to build up before breakdown in the stratosphere can remove them (and then there
is a whole new set of problems from CFC breakdown in the stratosphere).