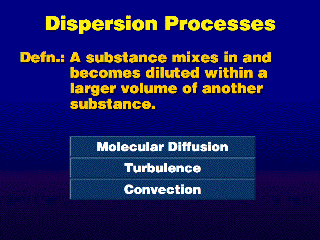
 Dispersion acts to mix pollution with clean ambient air, causing a dilution
of the pollution.
Dispersion acts to mix pollution with clean ambient air, causing a dilution
of the pollution. Dispersion acts to mix pollution with clean ambient air, causing a dilution
of the pollution.
Dispersion acts to mix pollution with clean ambient air, causing a dilution
of the pollution.
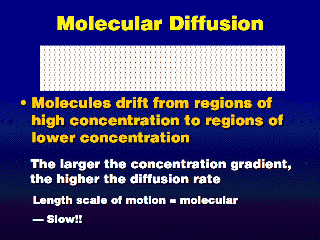 Pollution diffuses from highly polluted areas toward clean air. This
follows nature's tendency to smooth out irregularities and reach equilibrium.
At the molecular level, the mixing does not occur very quickly; in fact, it
slows down over time, because the speed of the diffusion slows down as the difference
in concentration between two points (the concentration gradient) decreases.
It could take several hours for a pollution release in one corner of an average
room to uniformly disperse throughout the room solely by molecular diffusion.
Pollution diffuses from highly polluted areas toward clean air. This
follows nature's tendency to smooth out irregularities and reach equilibrium.
At the molecular level, the mixing does not occur very quickly; in fact, it
slows down over time, because the speed of the diffusion slows down as the difference
in concentration between two points (the concentration gradient) decreases.
It could take several hours for a pollution release in one corner of an average
room to uniformly disperse throughout the room solely by molecular diffusion.
 Somewhat larger, but still random air motions. It resembles molecular diffusion,
but the scale of motion is much larger (centimeters in small areas, hundreds
of meters outdoors in the presence of strong winds). For a plume of smoke that
is being blown along by a breeze of clean air, there is turbulent mixing at
the edges of the plume. The concentration of pollution decreases as you move
laterally outward from the plume core; the overall concentration decreases downwind
of the smokestack as the plume "fans" out due to the turbulent mixing
along the edges.
Somewhat larger, but still random air motions. It resembles molecular diffusion,
but the scale of motion is much larger (centimeters in small areas, hundreds
of meters outdoors in the presence of strong winds). For a plume of smoke that
is being blown along by a breeze of clean air, there is turbulent mixing at
the edges of the plume. The concentration of pollution decreases as you move
laterally outward from the plume core; the overall concentration decreases downwind
of the smokestack as the plume "fans" out due to the turbulent mixing
along the edges.
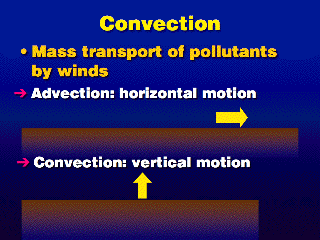 The air motions here are more organized than in turbulence; that is, there
is little or no randomness, just a determined motion in one direction. The extent
of the mixing in the horizontal is proportional to the wind speed. The extent
of the mixing in the vertical is controlled by the stability
of the atmosphere.
The air motions here are more organized than in turbulence; that is, there
is little or no randomness, just a determined motion in one direction. The extent
of the mixing in the horizontal is proportional to the wind speed. The extent
of the mixing in the vertical is controlled by the stability
of the atmosphere.