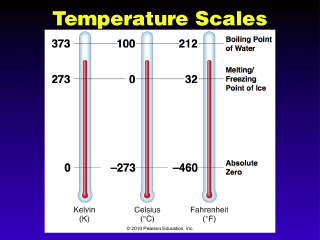
 "Absolute Zero" is the temperature where all molecular motion stops
(molecular speed is zero). There can be no values below this, so physicists
prefer this scale for energy equations, because energy based on temperature
cannot be negative (this scale, the Kelvin scale, is also called the
Absolute Scale for this reason).
"Absolute Zero" is the temperature where all molecular motion stops
(molecular speed is zero). There can be no values below this, so physicists
prefer this scale for energy equations, because energy based on temperature
cannot be negative (this scale, the Kelvin scale, is also called the
Absolute Scale for this reason).
This chart shows the correspondence
for the main temperature scales at various reference points ("room temperature"
is not really a reference point). Note that there are 100 Celsius degrees between
water freezing and boiling, 100 Kelvins (there are no Kelvin "degrees")
between these same points, and 180 Fahrenheit degrees from freezing to boiling.
Some stories: Celsius originally
had 0 C at water boiling and 100 C at water freezing, so his scale ran in reverse!
This is why you may see this scale called the Centigrade scale quite
often, because this is Celsius' original scale reversed to a more logical order.
Fahrenheit first used human body temperature as a reference point (100
F), which partially accounts for the odd temperature values for water freezing
and boiling. However, body temperature is actually 98.6 F (and 37 C, incidentally),
since Fahrenheit's measurements were not exactly accurate, and he was measuring
people's temperature in a place that is normally a little warmer than the standard
body temperature.
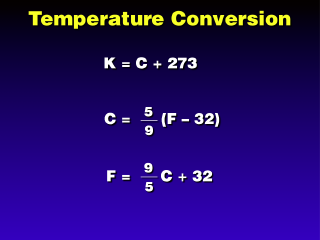 Looking back at our comparison of temperature scales, it appears that the
C and K scales both have 100 units between water freezing and boiling. So the
conversion is simply the addition or subtraction of 273.
Looking back at our comparison of temperature scales, it appears that the
C and K scales both have 100 units between water freezing and boiling. So the
conversion is simply the addition or subtraction of 273.
Going between F and C is a little
more complicated because the F scale has 180 degrees between water freezing
and melting. This is where the 5/9 or 9/5 factors come from.



 "Absolute Zero" is the temperature where all molecular motion stops
(molecular speed is zero). There can be no values below this, so physicists
prefer this scale for energy equations, because energy based on temperature
cannot be negative (this scale, the Kelvin scale, is also called the
Absolute Scale for this reason).
"Absolute Zero" is the temperature where all molecular motion stops
(molecular speed is zero). There can be no values below this, so physicists
prefer this scale for energy equations, because energy based on temperature
cannot be negative (this scale, the Kelvin scale, is also called the
Absolute Scale for this reason). Looking back at our comparison of temperature scales, it appears that the
C and K scales both have 100 units between water freezing and boiling. So the
conversion is simply the addition or subtraction of 273.
Looking back at our comparison of temperature scales, it appears that the
C and K scales both have 100 units between water freezing and boiling. So the
conversion is simply the addition or subtraction of 273.