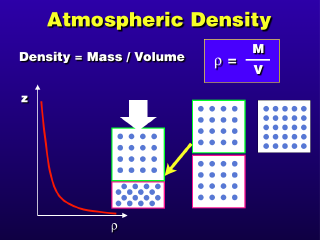
 Density is a measurement of the mass of a substance per unit volume
of the substance. The denser a substance is, the "heavier" it is for
a given volume. Note that the density resembles our measurement of concentration.
In fact, the concentration is really a measure of the density of the mixed-in
substance, without all the other stuff mixed with it.
Density is a measurement of the mass of a substance per unit volume
of the substance. The denser a substance is, the "heavier" it is for
a given volume. Note that the density resembles our measurement of concentration.
In fact, the concentration is really a measure of the density of the mixed-in
substance, without all the other stuff mixed with it.
The illustration shows how two similar
volumes with different numbers of molecules within have different densities.
The box with more molecules has them packed more closely together, so the number
of molecules per unit volume is greater than the box to its left. The bottom
set of boxes shows the effect of changing the volume of a sample of gas: if
you take the box on the left and compress it (make its volume smaller),
the density increases. Conversely, expanding the box on the right to
the larger volume on the left causes the density to decrease.This is mainly
a consideration for gases, because liquids and solids have no space between
their molecules that can be compressed or expanded (thus, their densities tend
to remain constant when external forces try to compress them).
The atmospheric density decreases
with increasing altitude, but not at a constant rate. It appears that the density
decreases rapidly with altitude in the lower atmosphere, then decreases at a
much lower rate in the upper atmosphere (the reason the atmospheric density
decreases with altitude is that the weight of the atmosphere above compresses
the atmosphere below, and the higher up one goes, the less atmosphere above
to compress the air at one's current location).
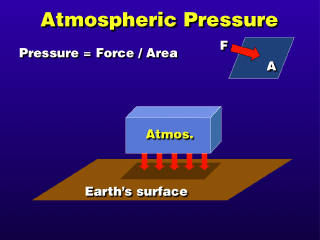 Pressure is the result of a force acting against an area of surface
(thus, the physical units of pressure are force/area). In the atmosphere, this
pressure is the result of the force of gravity causing the air to exert weight
against the ground. If we go to an altitude above the ground, the pressure
will be less because the weight of the atmosphere above is less (the atmosphere
below the chosen altitude does not figure into the pressure force, although
that part of the atmosphere is actually exerting a pressure force upward to
exactly balance the weight of the atmosphere above...which is why all of the
atmospheric gas molecules are not completely crashed onto the ground). From
this, we get the general relation that atmospheric pressure decreases as
the altitude increases.
Pressure is the result of a force acting against an area of surface
(thus, the physical units of pressure are force/area). In the atmosphere, this
pressure is the result of the force of gravity causing the air to exert weight
against the ground. If we go to an altitude above the ground, the pressure
will be less because the weight of the atmosphere above is less (the atmosphere
below the chosen altitude does not figure into the pressure force, although
that part of the atmosphere is actually exerting a pressure force upward to
exactly balance the weight of the atmosphere above...which is why all of the
atmospheric gas molecules are not completely crashed onto the ground). From
this, we get the general relation that atmospheric pressure decreases as
the altitude increases.
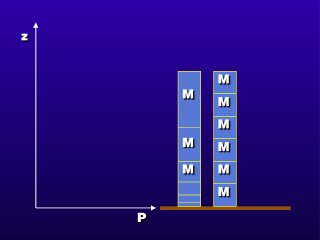 The weight of the atmosphere above compresses the atmosphere below. Because
the weight above a given altitude decreases as altitude increases, the degree
of compression decreases with increasing altitude. Since the compression of
the air causes its density to increase, we have relatively high atmospheric
density at low altitudes and lower densities above.
The weight of the atmosphere above compresses the atmosphere below. Because
the weight above a given altitude decreases as altitude increases, the degree
of compression decreases with increasing altitude. Since the compression of
the air causes its density to increase, we have relatively high atmospheric
density at low altitudes and lower densities above.
The column of air masses on the right
show that each mass occupies equal volumes. This would apply to water, which
does not compress under its own weight. Therefore, the density is constant throughout
a depth of water (the exception would be an immensely deep body of water, such
as an ocean, where the density of water at the bottom is actually somewhat greater
than at the top surface of the water body). The column at the left shows the
same masses for a column of air. We can see the varying degrees of compression,
due to the different amounts of air mass above pressing down on the rest of
the atmosphere.
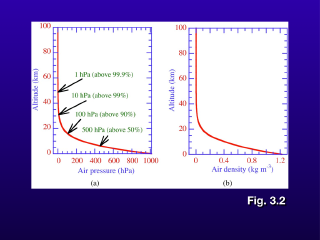
Both atmospheric pressure and
density decrease with increasing altitude, though the rate at which they
decrease also decreases with altitude, producing a curve instead of a straight
line on a vertical variation plot. The significance of the curve is that most
of the atmospheric mass in concentrated in the lowest part of the atmosphere
(about 80% of the total atmospheric mass is in the troposphere). Chemical
reactions occur at faster rates when the concentrations of the reactants are
high, so much of the atmospheric chemistry involved in air pollution will be
taking place in the lower part of the atmosphere.
 Given that about 80% of the atmospheric
mass is in the troposphere, it also means that most of the atmosphere to be
polluted will be in the troposphere, where we live and breathe. Thus, the most
significant air pollution problems will occur in the troposphere, and not just
because humans are living in that region of the atmosphere.
Given that about 80% of the atmospheric
mass is in the troposphere, it also means that most of the atmosphere to be
polluted will be in the troposphere, where we live and breathe. Thus, the most
significant air pollution problems will occur in the troposphere, and not just
because humans are living in that region of the atmosphere.



 Density is a measurement of the mass of a substance per unit volume
of the substance. The denser a substance is, the "heavier" it is for
a given volume. Note that the density resembles our measurement of concentration.
In fact, the concentration is really a measure of the density of the mixed-in
substance, without all the other stuff mixed with it.
Density is a measurement of the mass of a substance per unit volume
of the substance. The denser a substance is, the "heavier" it is for
a given volume. Note that the density resembles our measurement of concentration.
In fact, the concentration is really a measure of the density of the mixed-in
substance, without all the other stuff mixed with it. Pressure is the result of a force acting against an area of surface
(thus, the physical units of pressure are force/area). In the atmosphere, this
pressure is the result of the force of gravity causing the air to exert weight
against the ground. If we go to an altitude above the ground, the pressure
will be less because the weight of the atmosphere above is less (the atmosphere
below the chosen altitude does not figure into the pressure force, although
that part of the atmosphere is actually exerting a pressure force upward to
exactly balance the weight of the atmosphere above...which is why all of the
atmospheric gas molecules are not completely crashed onto the ground). From
this, we get the general relation that atmospheric pressure decreases as
the altitude increases.
Pressure is the result of a force acting against an area of surface
(thus, the physical units of pressure are force/area). In the atmosphere, this
pressure is the result of the force of gravity causing the air to exert weight
against the ground. If we go to an altitude above the ground, the pressure
will be less because the weight of the atmosphere above is less (the atmosphere
below the chosen altitude does not figure into the pressure force, although
that part of the atmosphere is actually exerting a pressure force upward to
exactly balance the weight of the atmosphere above...which is why all of the
atmospheric gas molecules are not completely crashed onto the ground). From
this, we get the general relation that atmospheric pressure decreases as
the altitude increases. The weight of the atmosphere above compresses the atmosphere below. Because
the weight above a given altitude decreases as altitude increases, the degree
of compression decreases with increasing altitude. Since the compression of
the air causes its density to increase, we have relatively high atmospheric
density at low altitudes and lower densities above.
The weight of the atmosphere above compresses the atmosphere below. Because
the weight above a given altitude decreases as altitude increases, the degree
of compression decreases with increasing altitude. Since the compression of
the air causes its density to increase, we have relatively high atmospheric
density at low altitudes and lower densities above. Given that about 80% of the atmospheric
mass is in the troposphere, it also means that most of the atmosphere to be
polluted will be in the troposphere, where we live and breathe. Thus, the most
significant air pollution problems will occur in the troposphere, and not just
because humans are living in that region of the atmosphere.
Given that about 80% of the atmospheric
mass is in the troposphere, it also means that most of the atmosphere to be
polluted will be in the troposphere, where we live and breathe. Thus, the most
significant air pollution problems will occur in the troposphere, and not just
because humans are living in that region of the atmosphere.