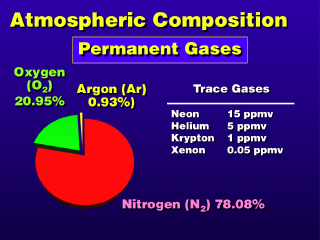
 The mixing ratios for these gases do not vary in our lifetimes; there may
be some variation over geological time (billions of years). The "other"
permanent gases are present in such low quantities, they are inconsequential
to human life.
The mixing ratios for these gases do not vary in our lifetimes; there may
be some variation over geological time (billions of years). The "other"
permanent gases are present in such low quantities, they are inconsequential
to human life. The mixing ratios for these gases do not vary in our lifetimes; there may
be some variation over geological time (billions of years). The "other"
permanent gases are present in such low quantities, they are inconsequential
to human life.
The mixing ratios for these gases do not vary in our lifetimes; there may
be some variation over geological time (billions of years). The "other"
permanent gases are present in such low quantities, they are inconsequential
to human life.
Alternate names for permanent gases are non-variable gases and fixed gases. In this sense, the "fixed" gases are not the same as the "fixed" air that was named by Joseph Black (which was actually carbon dioxide).
 Water vapor and carbon dioxide are the most important of the variable gases
because (1) they are the most abundant variable gases and (2) they both act
as greenhouse gases, due to their ability to absorb infrared (IR) radiation,
which causes the Earth's surface to be warmer than if there was no atmosphere.
Water vapor and carbon dioxide are the most important of the variable gases
because (1) they are the most abundant variable gases and (2) they both act
as greenhouse gases, due to their ability to absorb infrared (IR) radiation,
which causes the Earth's surface to be warmer than if there was no atmosphere.
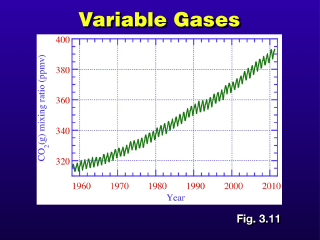 Carbon dioxide concentrations vary seasonally. We have also observed an overall upward trend in carbon dioxide concentration since industrialization in the 1700s, which is when carbon-based fossil fuels, such as coal, were used widely as an energy source. The correlation between this significantly increased use of fossil fuels and the the increase in atmospheric carbon dioxide is pretty strong, and is believed to be responsible for much of the global temperature increase in recent times.
Carbon dioxide concentrations vary seasonally. We have also observed an overall upward trend in carbon dioxide concentration since industrialization in the 1700s, which is when carbon-based fossil fuels, such as coal, were used widely as an energy source. The correlation between this significantly increased use of fossil fuels and the the increase in atmospheric carbon dioxide is pretty strong, and is believed to be responsible for much of the global temperature increase in recent times.
 There are non-varying trace gases; their mixing ratios are given in
the screen at the top of this page.
There are non-varying trace gases; their mixing ratios are given in
the screen at the top of this page.
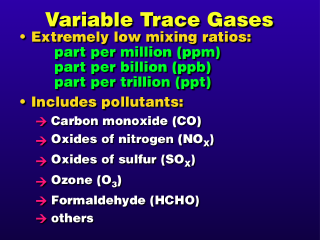
We are most interested in the variable
trace gases because many of them are pollutant gases. A few examples are: O3
(ozone), NOx (nitrogen oxides, where x = 1, 2, . . .), SOx
(sulfur oxides), CO (carbon monoxide), and so on.