Most environmental acids from anthropogenic sources are from atmospheric sulfur
(usually in the form of SOx). A substantial amount of environmental acidification
does come from NOx forming nitric acid (from combustion
sources of air pollutants).
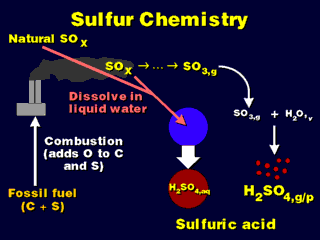 The main result of the sulfur chemistry is to produce sulfurous (H2SO3)
or, eventually, sulfuric (H2SO4) acid, which may or may not be dissolved
in water (technically, the liquid solutions of sulfuric/sulfurous acid and water
are called "sulfuric/ous acid solution" or "sulfuric/ous acid
in solution" while the straight "acid" designation would be the
gaseous forms of these chemicals).
The main result of the sulfur chemistry is to produce sulfurous (H2SO3)
or, eventually, sulfuric (H2SO4) acid, which may or may not be dissolved
in water (technically, the liquid solutions of sulfuric/sulfurous acid and water
are called "sulfuric/ous acid solution" or "sulfuric/ous acid
in solution" while the straight "acid" designation would be the
gaseous forms of these chemicals).
The "aq" subscripts denote substances dissolved in liquid water
or the liquid water itself (an "aqueous" solution); the "g"
subscripts denote substances existing as gas molecules; the "v"
subscript denotes water vapor (gaseous water).



 The main result of the sulfur chemistry is to produce sulfurous (H2SO3)
or, eventually, sulfuric (H2SO4) acid, which may or may not be dissolved
in water (technically, the liquid solutions of sulfuric/sulfurous acid and water
are called "sulfuric/ous acid solution" or "sulfuric/ous acid
in solution" while the straight "acid" designation would be the
gaseous forms of these chemicals).
The main result of the sulfur chemistry is to produce sulfurous (H2SO3)
or, eventually, sulfuric (H2SO4) acid, which may or may not be dissolved
in water (technically, the liquid solutions of sulfuric/sulfurous acid and water
are called "sulfuric/ous acid solution" or "sulfuric/ous acid
in solution" while the straight "acid" designation would be the
gaseous forms of these chemicals).