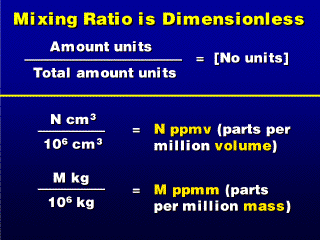
 We can compare either numbers of molecules, masses or volumes of gases when
determining mixing ratio. Because the units cancel out, yet are important because
the same amounts of gases produce different mixing ratios
depending on whether we are comparing masses or volumes,
we specify which by saying "parts per million volume" (ppmv)
or parts per million mass" (ppmm). When we see neither specification,
it's probably because the basis of comparison was a count of the numbers of
molecules. For gases at a given pressure, the volume taken up by a gas sample
will be directly proportional to the number of gas molecules in the sample ,
so in the absence of a volume or mass qualifier, assume the mixing ratio measurement
is a volume measurement.
We can compare either numbers of molecules, masses or volumes of gases when
determining mixing ratio. Because the units cancel out, yet are important because
the same amounts of gases produce different mixing ratios
depending on whether we are comparing masses or volumes,
we specify which by saying "parts per million volume" (ppmv)
or parts per million mass" (ppmm). When we see neither specification,
it's probably because the basis of comparison was a count of the numbers of
molecules. For gases at a given pressure, the volume taken up by a gas sample
will be directly proportional to the number of gas molecules in the sample ,
so in the absence of a volume or mass qualifier, assume the mixing ratio measurement
is a volume measurement.



 We can compare either numbers of molecules, masses or volumes of gases when
determining mixing ratio. Because the units cancel out, yet are important because
the same amounts of gases produce different mixing ratios
depending on whether we are comparing masses or volumes,
we specify which by saying "parts per million volume" (ppmv)
or parts per million mass" (ppmm). When we see neither specification,
it's probably because the basis of comparison was a count of the numbers of
molecules. For gases at a given pressure, the volume taken up by a gas sample
will be directly proportional to the number of gas molecules in the sample ,
so in the absence of a volume or mass qualifier, assume the mixing ratio measurement
is a volume measurement.
We can compare either numbers of molecules, masses or volumes of gases when
determining mixing ratio. Because the units cancel out, yet are important because
the same amounts of gases produce different mixing ratios
depending on whether we are comparing masses or volumes,
we specify which by saying "parts per million volume" (ppmv)
or parts per million mass" (ppmm). When we see neither specification,
it's probably because the basis of comparison was a count of the numbers of
molecules. For gases at a given pressure, the volume taken up by a gas sample
will be directly proportional to the number of gas molecules in the sample ,
so in the absence of a volume or mass qualifier, assume the mixing ratio measurement
is a volume measurement.