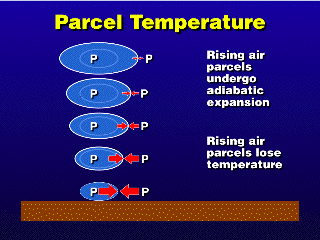
 Parcels moving up and down in the atmosphere experience changes in pressure
outside, which must be matched inside according to our definition of an
air parcel (p_in = p_out). Since no air can go in or out of the parcel,
the parcel must expand to reduce its interior pressure or compress
in order to increase its interior pressure. Thus, rising parcels expand,
and sinking parcels compress.
Parcels moving up and down in the atmosphere experience changes in pressure
outside, which must be matched inside according to our definition of an
air parcel (p_in = p_out). Since no air can go in or out of the parcel,
the parcel must expand to reduce its interior pressure or compress
in order to increase its interior pressure. Thus, rising parcels expand,
and sinking parcels compress.
The act of expanding or compressing a parcel changes the speed of the
molecules of air inside the parcel, so the temperature of the air inside
changes while a parcel rises or sinks. Since no heat is going in or out
of the parcel, the temperature changes occur adiabatically (if heat
was actually added or taken away to make the temperature go up or down,
respectively, we would call this a diabatic temperature change). Air
parcels rising or sinking in the atmosphere are generally assumed to be
losing or gaining temperature, respectively, by an adiabatic process.
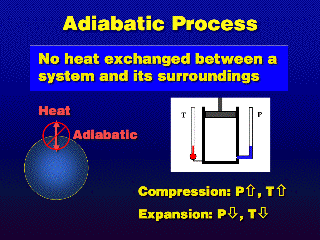 [Higher-level physics for those who are interested: We use the terms
"energy" and "heat" to mean the same thing, the "dQ"
term in the First Law of Thermodynamics (below). dQ represents the amount of
heat added/taken away from the system.
[Higher-level physics for those who are interested: We use the terms
"energy" and "heat" to mean the same thing, the "dQ"
term in the First Law of Thermodynamics (below). dQ represents the amount of
heat added/taken away from the system.
dU = dQ + dW
In an adiabatic process, dQ=0, so compressing or expanding the gases
adiabatically (which is the "dW" or "work" term) causes
a temperature change (via the "dU" or "internal energy"
term, where U is a direct function of temperature T in an ideal gas).]
You have probably experienced this phenomenon if you have pumped up a
bicycle tire or a rubber ball---it gets hot as you compress the air. If
you have sprayed an aerosol can, you may have noticed the can and the spray
become cold, due to the adiabatic expansion when you release the pressure
of the can's contents.
Play the QuickTime movie of an adiabatic compression
and expansion.
Putting this all together, since a rising parcel expands adiabatically, a rising
air parcel loses temperature; an adiabatically sinking parcel gains temperature
since it is compressing. For air that is dry (not enough water vapor to saturate
the air), it turns out that in Earth's troposphere, all air parcels are losing
or gaining temperature at the rate of 10°C per km change in altitude.
This rate of change of temperature is referred to as the Adiabatic
Lapse Rate*.
*In general meteorology, there is a case where the air
is saturated with water vapor and is rising/sinking pseudoadiabatically.
Because the condensation or evaporation of water while this is going on
is adding or removing (latent) heat, the lapse rate in these saturated air
parcels is altered. This Saturated Adiabatic Lapse Rate (4 to 10°C/km)
is smaller than the Dry Adiabatic Lapse Rate (10°C/km) we will be using
for all of our air pollution cases.



 Parcels moving up and down in the atmosphere experience changes in pressure
outside, which must be matched inside according to our definition of an
air parcel (p_in = p_out). Since no air can go in or out of the parcel,
the parcel must expand to reduce its interior pressure or compress
in order to increase its interior pressure. Thus, rising parcels expand,
and sinking parcels compress.
Parcels moving up and down in the atmosphere experience changes in pressure
outside, which must be matched inside according to our definition of an
air parcel (p_in = p_out). Since no air can go in or out of the parcel,
the parcel must expand to reduce its interior pressure or compress
in order to increase its interior pressure. Thus, rising parcels expand,
and sinking parcels compress. [Higher-level physics for those who are interested: We use the terms
"energy" and "heat" to mean the same thing, the "dQ"
term in the First Law of Thermodynamics (below). dQ represents the amount of
heat added/taken away from the system.
[Higher-level physics for those who are interested: We use the terms
"energy" and "heat" to mean the same thing, the "dQ"
term in the First Law of Thermodynamics (below). dQ represents the amount of
heat added/taken away from the system.