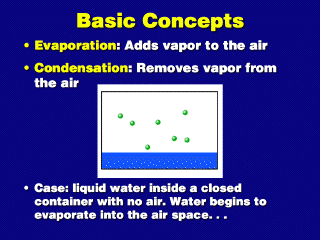
 In this system, there is air space (not necessarily with any other gases other
than vapor; it can be a vacuum) above some liquid water. At any time, a number
of liquid molecules jump off the surface and change into vapor molecules (evaporation),
while some vapor molecules simultaneously fall back and become liquid water
again (condensation).
In this system, there is air space (not necessarily with any other gases other
than vapor; it can be a vacuum) above some liquid water. At any time, a number
of liquid molecules jump off the surface and change into vapor molecules (evaporation),
while some vapor molecules simultaneously fall back and become liquid water
again (condensation).
Note that evaporation increases the
number of vapor molecules in the air while condensation reduces the number of
vapor molecules that are airborne.
 When there are few vapor molecules in the air space, the rate at which evaporation
takes place exceeds the rate of condensation, so net evaporation results. This
occurs when the air space is subsaturated, or has a vapor
pressure less than what it would be if the air was saturated.
When there are few vapor molecules in the air space, the rate at which evaporation
takes place exceeds the rate of condensation, so net evaporation results. This
occurs when the air space is subsaturated, or has a vapor
pressure less than what it would be if the air was saturated.
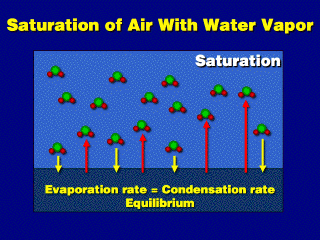 After a while, the air space contains a lot of vapor molecules. As the numbers
increase, the rate of condensation increases (the evaporation rate is constant,
and is directly dependent on temperature). Eventually, the evaporation and condensation
rates will be the same, and a steady-state condition is achieved---the vapor
pressure is constant and there is no net evaporation. This is the state
of saturation, and the vapor pressure at this point is the saturation
vapor pressure (e_sat).
After a while, the air space contains a lot of vapor molecules. As the numbers
increase, the rate of condensation increases (the evaporation rate is constant,
and is directly dependent on temperature). Eventually, the evaporation and condensation
rates will be the same, and a steady-state condition is achieved---the vapor
pressure is constant and there is no net evaporation. This is the state
of saturation, and the vapor pressure at this point is the saturation
vapor pressure (e_sat).
If the system's temperature was increased,
the liquid and vapor molecules start to bounce around with greater speed. This
makes more liquid molecules reach a state where they jump off and evaporate
(the absolute evaporation rate increases as temperature increases). With a higher
evaporation rate, we need a higher condensation rate in order to have equilibrium.
To get this higher condensation rate, there needs to be a greater number of
vapor molecules in the air space. Thus, as the temperature increases, the
saturation vapor pressure increases. Note that the saturation value is merely
a potential maximum amount of vapor, because at any one time,
the air could be subsaturated (e less than e_sat) or even supersaturated
(e greater than e_sat).



 In this system, there is air space (not necessarily with any other gases other
than vapor; it can be a vacuum) above some liquid water. At any time, a number
of liquid molecules jump off the surface and change into vapor molecules (evaporation),
while some vapor molecules simultaneously fall back and become liquid water
again (condensation).
In this system, there is air space (not necessarily with any other gases other
than vapor; it can be a vacuum) above some liquid water. At any time, a number
of liquid molecules jump off the surface and change into vapor molecules (evaporation),
while some vapor molecules simultaneously fall back and become liquid water
again (condensation). When there are few vapor molecules in the air space, the rate at which evaporation
takes place exceeds the rate of condensation, so net evaporation results. This
occurs when the air space is subsaturated, or has a
When there are few vapor molecules in the air space, the rate at which evaporation
takes place exceeds the rate of condensation, so net evaporation results. This
occurs when the air space is subsaturated, or has a  After a while, the air space contains a lot of vapor molecules. As the numbers
increase, the rate of condensation increases (the evaporation rate is constant,
and is directly dependent on temperature). Eventually, the evaporation and condensation
rates will be the same, and a steady-state condition is achieved---the vapor
pressure is constant and there is no net evaporation. This is the state
of saturation, and the vapor pressure at this point is the saturation
vapor pressure (e_sat).
After a while, the air space contains a lot of vapor molecules. As the numbers
increase, the rate of condensation increases (the evaporation rate is constant,
and is directly dependent on temperature). Eventually, the evaporation and condensation
rates will be the same, and a steady-state condition is achieved---the vapor
pressure is constant and there is no net evaporation. This is the state
of saturation, and the vapor pressure at this point is the saturation
vapor pressure (e_sat).