 A millibar (mb) is 1/1000 of a bar of pressure. A bar of pressure is
approximately the same as the average atmospheric pressure at sea level.
A millibar (mb) is 1/1000 of a bar of pressure. A bar of pressure is
approximately the same as the average atmospheric pressure at sea level. A millibar (mb) is 1/1000 of a bar of pressure. A bar of pressure is
approximately the same as the average atmospheric pressure at sea level.
A millibar (mb) is 1/1000 of a bar of pressure. A bar of pressure is
approximately the same as the average atmospheric pressure at sea level.
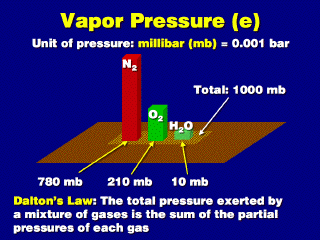
The weight of the entire atmosphere
exerts a pressure of about 1000 mb against the ground at sea level (1013.2 mb
average, to be more exact). This total weight consists of the sum of the weights
of all the various gases mixed together. Let's assume that the air consists
mostly of nitrogen, oxygen, and water vapor.
Dalton's Law of Partial Pressures says that the sum of the partial pressures of each gas in a mixture of gases equals the total pressure exerted by the entire mixture. This is because each molecule exerts a given amount of force from its collision with the walls of the containment vessel, or its individual weight pressing against the ground. So the pressure just from one particular gas must be proportional to the number of molecules of that gas, or its partial pressure is the same portion as the fraction of the total amount of all the gases mixed together.
Separating each gas and assuming 78% of the mixture is nitrogen, 21% is oxygen, and the remaining 1% is water vapor, we take the same same proportions of the total pressure: 780 mb of nitrogen, 210 mb of oxygen, and 10 mb of water vapor.
This 10 mb of vapor is the vapor
pressure (e). As the percent portion of atmopshere that is water vapor increases,
the vapor pressure increases proportionally. Thus, vapor pressure is an exact
measurement of the amount of vapor in the air (as such, it can be converted
into a number of vapor molecules per unit volume of air).