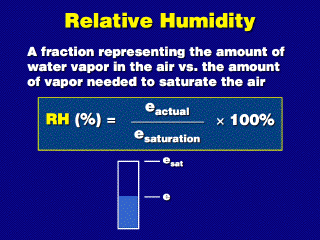
 Saturation vapor pressure is the potential maximum vapor pressure
for air at some temperature (ignoring the possibility of supersaturation).
At any one time, the actual vapor pressure e could be anything from 0
to e_sat. The relative humidity tells us how close we are to saturation;
e.g., if we have a third of the amount of vapor needed for saturation, the RH
is 33%. If the air is saturated, e = e_sat and RH is 100%.
Saturation vapor pressure is the potential maximum vapor pressure
for air at some temperature (ignoring the possibility of supersaturation).
At any one time, the actual vapor pressure e could be anything from 0
to e_sat. The relative humidity tells us how close we are to saturation;
e.g., if we have a third of the amount of vapor needed for saturation, the RH
is 33%. If the air is saturated, e = e_sat and RH is 100%.
If we are simply told the value of
the RH, we do not directly know what is the actual amount of vapor in the air
in terms of vapor pressure. To get that, we would need to dtermine e_sat
and do some algebra. For everyday use, the value of e is not very useful
because as humans, we are probably more interested in the rate of (net) evaporation
of water. The evaporation of water is what allows us to regulate our body temperature
(perspiration). The rate of evaporation is important for agriculture, since
the faster the evaporation of water from the ground, the more frequently the
ground must be irrigate.
The relative humidity is correlated
to evaporation rate: as relative humidity increases, the evaporation rate
decreases (and the net evaporation rate is zero at RH = 100%). This allows
us to take into account the temperature effect, which produces larger RH's at
lower temperatures for the same vapor pressure, thus reducing the evaporation
rate as the temperature decreases. (Note: Here, we are referring to the net
evaporation rate as being related to relative humidity. The actual, absolute
evaporation rate is dependent on the temperature---the
higher the temperature, the higher the absolute evaporation rate, which
in turn results in a higher saturation vapor pressure, if an infinite supply
of water is present.)



 Saturation vapor pressure is the potential maximum vapor pressure
for air at some temperature (ignoring the possibility of supersaturation).
At any one time, the actual vapor pressure e could be anything from 0
to e_sat. The relative humidity tells us how close we are to saturation;
e.g., if we have a third of the amount of vapor needed for saturation, the RH
is 33%. If the air is saturated, e = e_sat and RH is 100%.
Saturation vapor pressure is the potential maximum vapor pressure
for air at some temperature (ignoring the possibility of supersaturation).
At any one time, the actual vapor pressure e could be anything from 0
to e_sat. The relative humidity tells us how close we are to saturation;
e.g., if we have a third of the amount of vapor needed for saturation, the RH
is 33%. If the air is saturated, e = e_sat and RH is 100%.