

Radiation moves as packets of pure energy propagating through space or within a medium at the speed of light or close to it (3 X 108 m/s or 186,000 mph). Conduction and convection both need some substance, such as air, to transfer energy; but, radiation needs no transfer medium.
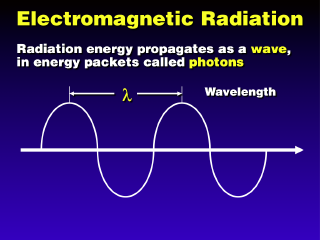 Electromagnetic radiation (EMR) acts both as a wave and as a particle, even
though physically it has no dimensions or mass.
Electromagnetic radiation (EMR) acts both as a wave and as a particle, even
though physically it has no dimensions or mass.
As a wave, EMR can be characterized by some wavelength, the distance between adjacent wavecrests. Various different types of EMR (as seen below) correspond to various different types of EMR.
The significance of the photon
is that the amount of energy contained in each photon is inversely proportional
to the wavelength of the radiation. In fact, this energy E is equal to hc/wavelength,
where h is the Boltzmann constant and c is the speed of light.
 The most important part of the entire spectrum of wavelengths is the UV-visible-IR
range, which is where most of the energy from the sun is emitted.
The most important part of the entire spectrum of wavelengths is the UV-visible-IR
range, which is where most of the energy from the sun is emitted.
Ultraviolet (UV) is short enough wavelength that each photon has sufficient energy to cause damage to living tissue. Fortunately, most of the solar UV is absorbed by oxygen and ozone in the upper atmosphere.
Visible light (so-called because our eyes are sensitive to just these wavelengths and our brain uses the signal from the eyes to form an image) is not absorbed by the atmosphere, so visible images can travel through the air.
Infrared (IR) is not visible,
but often felt as "heat" because our skin and other objects can absorb
the IR energy and heat up as a result.